Workpackages
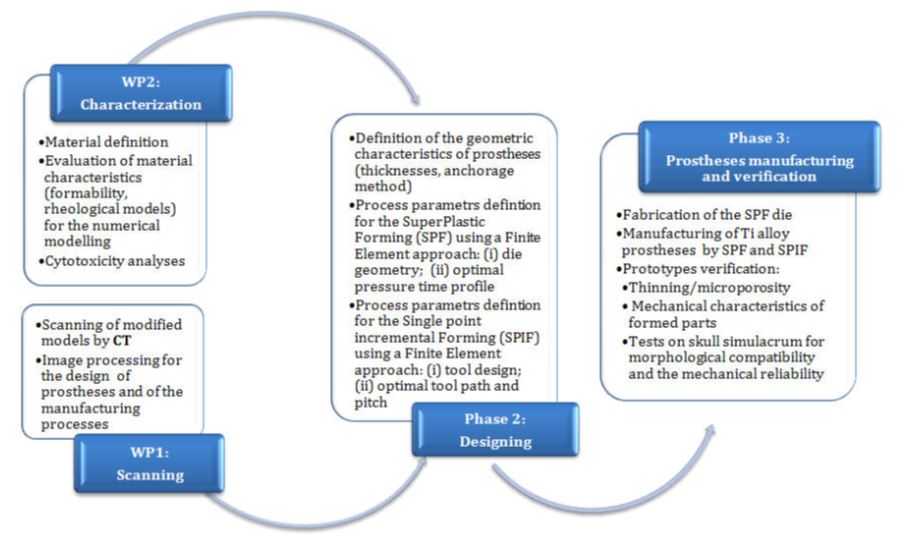
PHASE 1 requires the implementation of two work packages (WP):
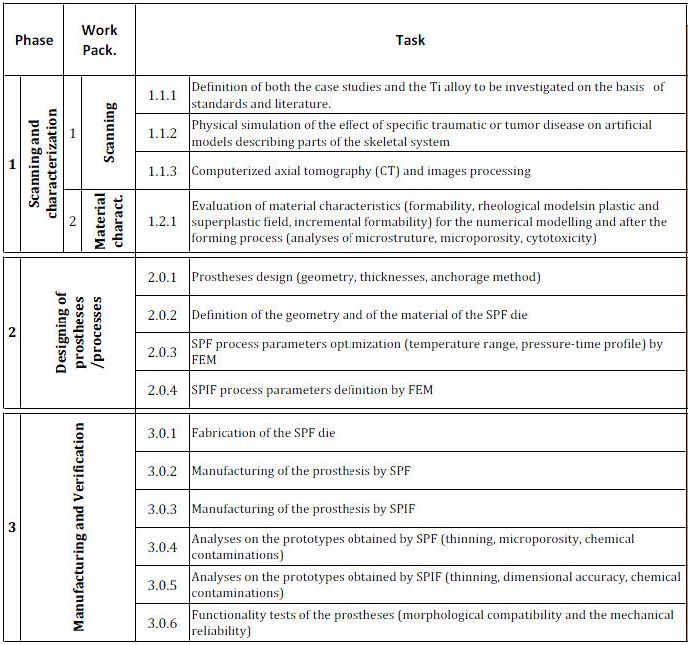
- in the WP-1 (Scanning) information in terms of diagnostic images through CT technique of artificial models of the skeletal system (skull) will be obtained; such models will be previously modified to simulate the effect of specific trauma or tumors; the images will be processed by means of specific software in order to virtually reconstruct the missing part and to make such information available for the prostheses design and the production process.
- in the WP-2 (Characterization), the material characterization will be carried out in order to identify the formability limits and define rheological models to be used for describing the plastic and superplastic material behaviour; such data are necessary for the numerical simulations aimed at both designing the prostheses and at defining the parameters of the two forming processes. Cytotoxicity analyses on specimens obtained by SPF and SPIF will be finally carried out in order to investigate the effect of the adopted manufacturing processes on the prostheses biocompatibility (possible alpha case creation).
In the PHASE 2 (Designing), carried out using a numerical approach based on the Finite Element Method (FEM), both the geometric characteristics of the case studies (thicknesses, anchorage method) and the process parameters of the two manufacturing processes (SPF and SPIF) will be defined; in particular, in order to find the most suitable one according to the specific prosthesis, the two processes will be compared.
The PHASE 3 (Prostheses manufacturing and verification) consists of forming tests aimed at producing the prostheses and testing the functionality of the formed components: prototypes, obtained by SPF and SPIF, will be analyzed in terms of thinning and will be sectioned in order to perform macro-and micro-optical analyses. In order to validate the whole procedure, in this phase tests aimed at verifying the effective compliance of the prosthesis to the bone defect created on artificial model will be executed: the morphological compatibility and the mechanical reliability will be thus verified. The prosthesis will be connected to the same skull models subjected to CT in the 1st phase (WP1). Thereafter the ability of the simulacra to fill the bone defects and their strength will be evaluated by tests on properly equipped machines.
Below, the list of tasks composing each WP is shown:
The network composition was designed considering that all the RUs already hold both the expertise and the equipment required for the experimental investigation.
Below a brief description of the expertise areas and the role of each research unit in the research project.
RU1 – POLITECNICO DI BARI
RU1’s researchers have wide experience both in the field of: (i) sheet forming process using flexible media; (ii) innovative materials and process for the manufacturing of complex shaped parts.
The above mentioned research topics are confirmed by several publications and by the National and Regional research projects. The experimental activities scheduled in the RP will be in fact developed in laboratories funded by the Apulia Region (Advanced Forming & Manufacturing Laboratory of TRASFORMA) and MIUR through PON projects (Laboratory of Physical Simulation of Technological Processes).
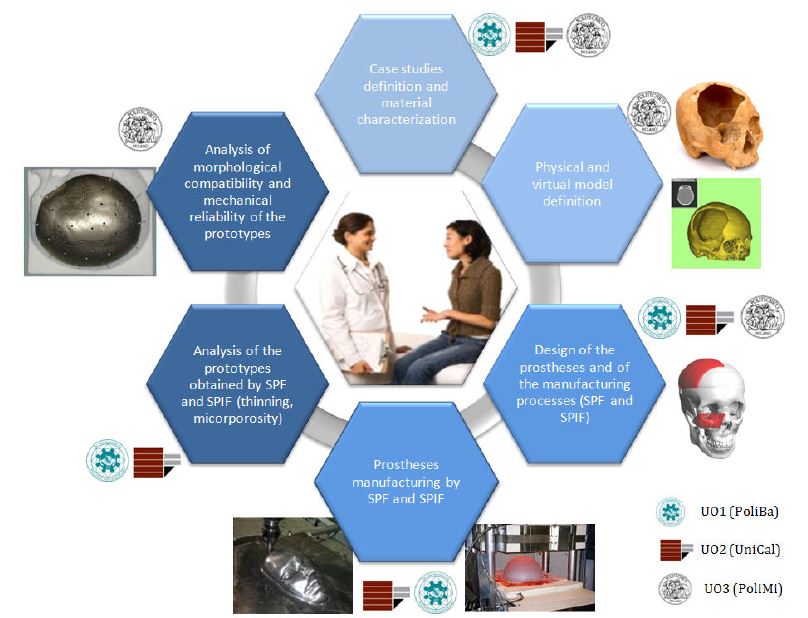
Together with the other RUs, RU1 will define the material and the prostheses to be investigated; both alloy biocompatibility and clinic aspects will be considered during this task. In the following, RU1 will analyse the mechanical behaviour of the used Ti alloy executing: (i) tensile tests in the range of 100-950°C by using the physical simulator Gleeble 3180 (high temperature and vacuum conditions); (ii) free inflation and closed die tests aimed at evaluating the maximum strain, the optimal strain rate and temperature range for the SPF process (such tests will be carried out both with and without a back pressure in order to investigate the effect on the cavitation and on the alpha-case extension). RU1 and RU2 will work together for the definition of material formability by means of the optical measurement system ARAMIS. RU1 will be involved in the SPF process design (FEM approach) and prosthesis manufacturing; finally, RU1 will conduct with RU2 and RU3 the simulacrum validation from geometrical, qualitative and microstructural point of view.
RU2 – UNIVERSITA’ DELLA CALABRIA
RU2 includes researchers of 3 scientific research fields, which range from manufacturing to bio-engineering and design. Concerning the manufacturing field, RU2 holds an intensive experience on the SPIF feasibility and reliability, by using general purpose machines for producing components with both conventional materials and lightweight alloy (at different temperature and speed levels). According to that, RU2’s researchers will be involved in the design, set up and validation of SPIF process to bio-engineering applications. From a bio-engineering point of view, RU2 holds competences on computational and numerical analysis on endo-prosthesis anchoring systems and on the influence of geometric configurations on skeletal structures.
The RU2’s CAD expertise will support the geometries optimisation both for prosthesis and dies needed by SPF operation. According to that, RU2’s expertise will be involved in the RP to complete and integrate RU3 in computer aided and FEM based designing and modelling activities and to define a flexible process to manufacture the prosthesis in simultaneous and integrated way with RU1. With respect to the RU1, the attention will be focused on that process able to ensure a short time to market, such as the SPIF.
Finally, in agreement with all RUs, RU2 will define the case studies and will analyse the formability limits of the investigated materials.
RU3 – POLITECNICO DI MILANO
RU3’s expertise is on the reconstruction of virtual models of parts of the skeletal system, starting from diagnostic images of the patient, ether for their usage in finite element models or for the reconstruction of physical models for surgical planning and on the pre-clinical evaluation of implantable devices, both through numerical and experimental techniques.
In the specific project, RU3 will get cranium artificial models that will be modified in order to simulate bone defects at the bone cranium level, zigomatic bones and temporo-mandibular bones. These models will be then subjected to CT scanning at the Galeazzi orthopaedic Institute of Milan, which RU3 has an active research agreement with: the images will be then elaborated via specific software to virtually reconstruct the missing part of the bony structures using a mirroring technique. At the meantime, RU3 will take care of cytotoxicity analyses (made in an external supplier laboratory) on specimens obtained through SPF/SPIF technology, in order to assess if any variation in the biocompatibility of the Ti alloy is introduced by the specific manufacturing process. The obtained virtual model will be then used by RU1 and RU2 for the manufacturing of the prosthesis Ti prototype. Once the prosthesis prototypes will be received from RU1 and RU2, they will be mounted by RU3 on the cranium models in order to assess the morphological compatibility; later, the cranium-prosthesis complex will be mounted on a testing machine at RU3 laboratory, using a purposely designed setup, for an experimental evaluation of the mechanical reliability of the construct.
At this stage of RP an in-depth planning of both the interactions and the procedures to implement them was performed. This is of fundamental importance considering the multidisciplinary nature of the RP and the request to create an expertise network whose nodes are represented by the three RU. This was considered strategic for an effective project management, i.e. able to ensure a successful progress of the scheduled activities.
In order to make easier the results sharing and to coordinate the activities development, web meetings will be organized at the end of each task of the RP. In this way, each intermediate result will be easily transferred and shared by all partners with regularity and high frequency (less than yearly). In fact, the progress of research activities is considered to be based on the discussion and the interaction between the different RUs. Each RU implements both individual and shared activities (based on the specific area of expertise). In order to go ahead with such a complex-structure RP, a leading RU was identified for each task, who is also responsible for the coordination of both the information exchange and the WM organization.
It was considered, in fact, that the research activities proposed in the present RP are based on the integration and the sharing of the RUs composing the network. Each RU can carry out both individual (based on its own expertise) and shared activities: in general, everything that is related with the design, testing and analysis of SPF and SPIF manufacturing will be coordinated by RU1 and RU2; similarly, physical simulation of the trauma and the final assessment on the bone simulacra will be coordinated by RU3. At the same time, however, several tasks will be developed in a synergic way among the RUs. For instance; the selection phase of the case studies and materials, with the relative mechanical technological and metallurgical characterization (1.2.1); the prostheses and dies design (2.0.1); the prostheses manufacturing (3.0.3); the prototypes validation from both the technological (3.0.4 and 3.0.5) and the bio-medical (3.0.6) point of view.
According to that, the mobility periods were temporally placed in order to match with the above mentioned activities, which are considered strategic for the RP. In fact, the above mentioned activities determine the achievement of important results for the RP progress; at the same time they are based on specific skills that can be shared just through the mobility: in such a way the mobility periods will contribute to create a common knowledge base between medicine and engineering (which is one of the goals of this RP); in addition, the contract staffs involved in these exchanges will make easier the management of the above mentioned strategic activities, while increasing their background.